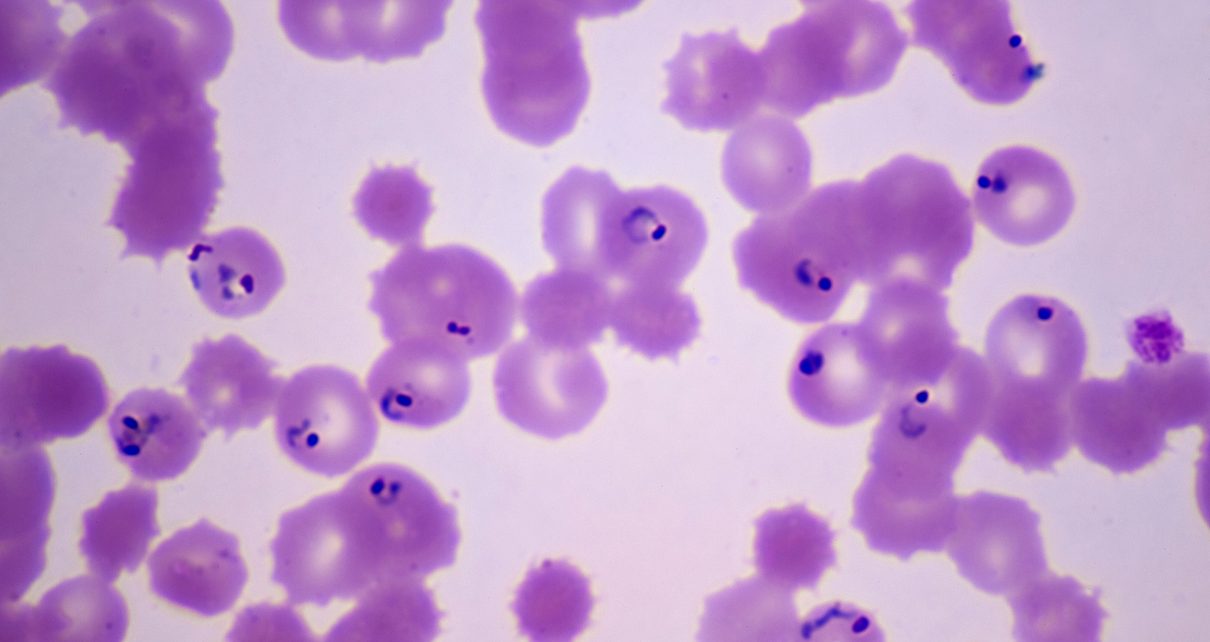
Malaria struck an estimated 228 million people worldwide in 2018. Yet questions remain about how the mosquito-borne malaria parasite, Plasmodium, infects humans—and how antimalarial-drug-resistance genes spread. Different strains of the parasite can exchange genes with one another when they reproduce sexually inside an individual mosquito, and the resulting mixed strains infect humans through the mosquito’s bite. A new study paints a detailed picture of how Plasmodium trades genes, and it finds that all the genetic diversity within an actively infected human host—up to 17 parasite strains—can come from just one bite. The work was published in January in Cell Host and Microbe.
Plasmodium spends part of its life cycle in humans and part in mosquitoes. In the mosquito, it reproduces, mixing and matching genes. Until now, the most efficient way to study Plasmodium‘s genetic diversity was to grind up whole mosquitoes and sequence the mix. The new technique lets scientists determine whether a patient’s particular parasites were the product of reproduction within a single mosquito or were introduced separately by different ones.
The researchers collected blood from patients at a hospital serving different villages in Malawi, then sequenced genomes of the parasites found in infected blood cells. Based on the parasites’ intermingled genomes, the researchers found that nearly all the infections studied likely came from an individual bite.
“Using single-cell sequencing of parasites from whole populations of infected individuals, we could really start to see for the first time how people are getting infected with malaria,” says Ian Cheeseman, a parasitologist at Texas Biomedical Research Institute and senior author of the new study. “Sometimes absolutely staggering amounts of genetic diversity are being transmitted in a single mosquito bite.”
The findings are consistent with what Dyann Wirth, an infectious disease researcher at Harvard University specializing in parasites, who was not involved in the new study, had suspected based on earlier research. She calls the work “an important technical breakthrough that will allow a much deeper understanding of malaria transmission and recombination.”
This technique can also indicate where infections are coming from. When eradication efforts reduce malaria cases in a given area, analyzing blood cells from those who still get sick can reveal if the infected mosquitoes came from afar or if local elimination was incomplete, explains Edward Wenger, director of global health research at the Institute for Disease Modeling in Bellevue, Wash., who was not involved in the study. The method could also help researchers track the proliferation of drug-resistance mutations. Finding these mutations—and containing their spread—is a critical public health strategy for preserving drugs’ effectiveness.