The ongoing tragedy of the COVID-19 pandemic afflicts every corner of the world. Vaccines may be our best hope for a safe return to workplaces, parties, stores and schools, but even if all leading vaccine candidates are protective, the British charity Oxfam estimates that nearly two thirds of the world’s population will not have access until at least 2022. We suggest a scalable alternative that may prevent morbidity and mortality from Covid-19 in the meantime: the common cold.
Many different studies have shown that infection with one of the seasonal human coronaviruses (shCoVs) responsible for common colds confers a cross-reactive T-cell immune response to SARS-CoV-2, and on September 17, the British Medical Journal published an editorial speculating that “preexisting immunity” to SARS-Cov-2 may result from T cell cross-reactivity.
Might common colds explain a great deal of preexisting immunity, and if so, could the responsible virus help save lives during the current pandemic?
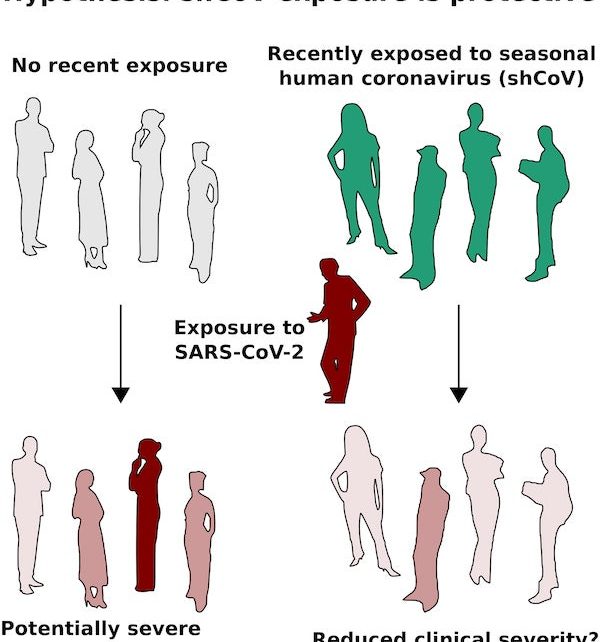
We currently lack direct evidence that exposure to any of the four “common cold” coronaviruses protects against severe COVID-19. However, searching for that evidence should be a high priority. Just as Efiguredward Jenner observed that cowpox exposure seemed to protect against smallpox and leveraged that discovery to save lives, future studies demonstrating even a small reduction in clinical severity of COVID-19 could enable safe, highly scalable, community-driven shCoV transmission programs (Fig. 1). Many people would welcome common cold symptoms from a virus they have previously endured in exchange for even a 10–20 percent reduction in the risk of severe illness or death.
Several key findings build the case for testing clinical protection. First, while acquired immunity to shCoVs is short–lived, allowing them to infect us again and again, it can protect against related coronaviruses. Second, hundreds of shCoV components detected by T cells are similar to those of SARS-CoV-2. Indeed, studies by immunologists such as Daniela Weiskopf and colleagues Alessandro Sette and Shane Crotty, the Karolinska COVID-19 study group and many others have identified T cells that react to SARS-CoV-2 in samples from people never exposed to the virus; many of these respond to components matching shCoVs. Third, the strength of T cell reactivity in people recovering from COVID-19 appears to be inversely proportional to disease severity; while there are exceptions, most people with a strong T cell response experience mild symptoms or none at all. Finally, common cold shCoVs are considered among the safest of viruses; as annoying as a stuffy nose can be, reports of serious complications appear rare, even though just about everyone has been infected.
While deliberate shCoV transmission programs would be highly premature, there are several ways for scientists to better determine whether common cold exposure could help save lives that merit serious consideration and debate.
First, samples from large numbers of COVID-19 cases of varying severity could be tested for recent exposure to the four shCoVs. Second, scientists could test participants in vaccine trials for recent shCoV exposure; this should arguably be done in any case because protection might otherwise confound the results of the vaccine study. Third and more ambitiously, a field trial could deliberately expose thousands of people to one of the shCoVs or to a common-cold rhinovirus as a control, then analyze the severity of COVID-19 in those participants who naturally become infected, just like a vaccine trial. People with comorbidities that put them at risk of a more serious disease course from shCOV or rhinovirus exposure, as well as anyone with household members who are similarly vulnerable, would need to be excluded.
Finally, and most controversially, healthy volunteers could be exposed to one of the four shCoVs or an unrelated mild rhinovirus causing similar symptoms, then challenged with SARS-CoV-2 a few weeks later to measure the extent of protection. Volunteers would need to be young with no comorbidities; their expenses and medical costs would need to be covered and a minimum wage provided.
Much of our case for studying the impact of shCoV exposure on COVID-19 severity relies on the premise that deliberate transmission of a protective common cold would be safe and rapidly scalable. To support this premise, we lay out one possible deliberate shCoV transmission program.
Communities encouraging person-to-person common cold transmission during a pandemic would need to ensure that donors do not have SARS-COV-2. Unfortunately, tests alone aren’t sensitive enough, especially early during infection. Isolating future donors before and after deliberate shCoV infection would limit their potential exposure to SARS-CoV-2 and ensure that testing is as sensitive as possible. In one implementation, shCoV donors could complete a 14-day (or longer) isolation during which they would be exposed to the common cold virus via Q-tip by another donor who has repeatedly tested negative for SARS-CoV-2 (Fig. 2).
Assuming that the typical donor can inoculate 100 recipients (10 of whom become next-generation donors) and that protection lasts for a year, the total material cost of our example approach would sum to approximately 26 SARS-CoV-2 tests plus Q-tips and plastic bags per thousand people (Fig. 2). For comparison, India is currently performing about 80 tests per thousand residents per year. Most nations could protect their most vulnerable with shCoVs within weeks and establish clinical protection across their populations within months, buying time until a more protective vaccine is available.
Importantly, deliberate shCoV transmission should face few if any regulatory delays. The ubiquity of common- cold coronaviruses combined with the dearth of reportedly serious complications attests to their safety; just as importantly, naturally occurring viruses are unlikely to suffer from regulatory delays. Before the chickenpox vaccine was introduced in the United States in 1995, there was no effort to regulate the deliberate transmission of chickenpox to children.
National agencies, international NGOs or local clinics helping to coordinate safe person-to-person shCoV transmission would be performing testing to prevent accidental transmission of SARS-CoV-2, not manufacturing, distributing or administering a drug. Once initiated by a community, common-cold transmission could scale rapidly: given the assumptions above, a single initial donor could help shield a million people from severe COVID-19 in 34 days.
Today, we know that recent exposure to common-cold coronaviruses confers T cell cross-reactivity against components of SARS-CoV-2, and that people with stronger T cell responses tend to experience fewer COVID-19 symptoms. To repeat, we lack direct evidence that recent shCoV exposure can reduce disease severity. We believe the scientific, ethical, and historical case for determining whether the common cold could save lives merits swift and thorough investigation.