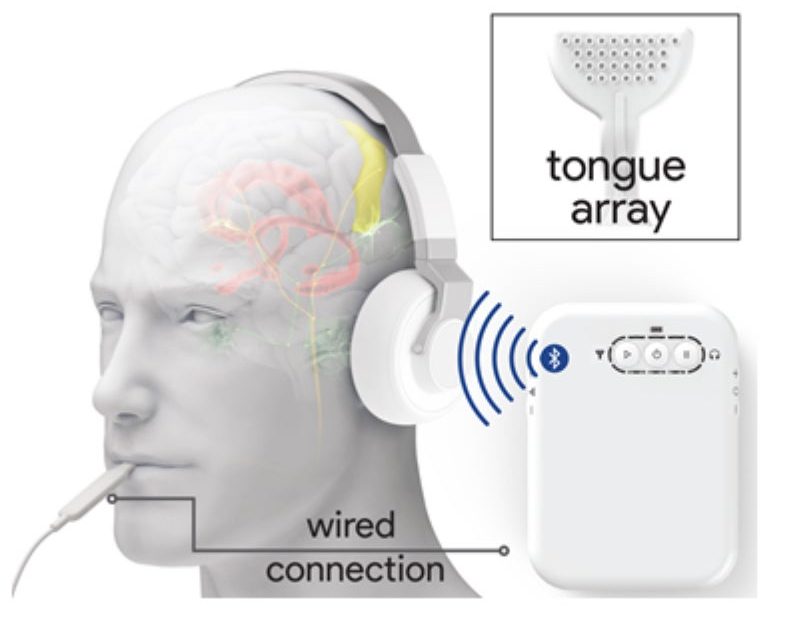
Tinnitus, the perception of phantom noises in the absence of actual sound, affects millions of people around the world. According to one recent assessment, approximately one in 10 adults in the U.S. experiences tinnitus—and in nearly a quarter of these individuals, symptoms last for more than 15 years. Those with tinnitus can also experience complications such as difficulty focusing, fatigue, anxiety and an overall reduction in the quality of life.
Psychological interventions such as cognitive-behavioral therapy can help lessen the distress, but to date, no drug or medical device has been shown to reliably improve this condition. Now researchers have inched closer to making a treatment for tinnitus a reality. According to a new study, published today in Science Translational Medicine, a noninvasive device that applies a technique known as bimodal neuromodulation, combining sounds with zaps to the tongue, may be an effective way to provide relief to tinnitus patients.
According to study co-author Hubert Lim, an associate professor of biomedical engineering and otolaryngology at the University of Minnesota, this treatment targets a subset of brain cells that are firing abnormally. Through studies in both humans and animals, Lim’s team and others previously reported that electrically stimulating touch-sensitive neurons in the tongue or face can activate neurons in the auditory system. Pairing these zaps with sounds appears to rewire brain circuits associated with tinnitus.
The technique developed by Lim and his colleagues is designed to promote the activation of brain circuits in response to many different sounds to drown out phantom noise. “The idea is that eventually your brain gets sensitive to many different things,” Lim explains. “In a way, you have suppressed the tinnitus neurons but only by elevating the other neurons.” Another group led by Susan Shore, a professor of otolaryngology at the University of Michigan, developed a similar device with a different approach: instead of increasing sensitivity to a broad spectrum of sounds, the team’s method pairs a sound that matches the phantom one heard by patients with a specifically timed electrical pulse to the head or neck. In a 2018 study that included 20 people with tinnitus, Shore’s team reported that this technique was effective in reducing the loudness and intrusiveness of the subjects’ tinnitus. “You can think of it as two ways to treat tinnitus,” Lim says. “One is you can try to find [the tinnitus cells] and shut them down. Our approach is to make everything in the auditory system much more hyperactive to everything but the tinnitus.”
To examine the efficacy and safety of their device, Lim and his colleagues conducted a randomized, double-blinded exploratory study with 326 adults who had chronic tinnitus at two sites: St. James’s Hospital in Ireland and the Tinnitus Center at the University of Regensburg in Germany. Participants were instructed to use the device for 60 minutes daily for 12 weeks. They were divided into three groups—each of which received slightly different treatments that varied by the type of sound used, the timing of electrical pulses, and the delay between the sound and the stimulation. The study was funded by Neuromod Devices, a Dublin-based company, where Lim is chief scientific officer, that is developing and selling the bimodal neuromodulation device.
Results showed that 84 percent of participants completed the 12-week regimen. Afterward, approximately 81 percent of treatment-compliant participants exhibited improvement in psychosocial variables such as the ability to concentrate or sleep, along with lower levels of anxiety and frustration and better quality of life. In around 77 percent of the group, this improvement persisted a year later. Also, 66 percent of participants reported feeling that they had benefited from the device. There were no significant differences in these measures among the three groups.
“The study is very thorough and comprehensive,” says Richard Tyler, an audiologist at the University of Iowa, who was not involved in the new study. “Given that, at this point, there is no pill or no surgery available for tinnitus, this work is very important.” He adds that the investigation had some notable shortcomings, however. The most concerning was the lack of a control condition in which some participants would not receive any therapeutic stimulation to rule out placebo effects. Another limitation was that the authors did not report whether the subjects experienced a reduction in tinnitus—actual changes in the perceptions of the phantom sounds. “You have tinnitus, and you have your reactions to tinnitus. Those are two different things,” Tyler says. “If you’re going to try and decrease the tinnitus, then you should be measuring the tinnitus.”
According to Lim, his group chose to focus on how the study participants reacted to tinnitus because patients’ auditory perceptions may vary, depending on how they are affected by the condition. The team did, however, measure perceptual changes and plans to present those findings in a subsequent paper.
“I was impressed with the improvements measured in the patients,” says Rilana Cima, a psychologist at Maastricht University in the Netherlands, who was not involved in this research but is currently collaborating with some of the co-authors on another study. Although the approach seems promising, it would be useful to see whether a group unaffiliated with the company developing the device would be able to replicate these results, she adds. “I would advise, before we start producing these things en masse, to do that first.”
Neuromod’s bimodal neuromodulation device is currently available through physicians in Ireland and Germany for prices from €2,500 to €2,750 (around $2,900 to $3,200). According to Lim, the company is also seeking approval from the Food and Drug Administration to make the treatment available in the U.S. His group also plans further experiments to examine the mechanism underlying its effectiveness. “At this stage, we can say that bimodal stimulation is changing things in the brain,” Lim says. “The next step is to do brain imaging [in humans] and animal experiments to really figure out what has changed in the brain.”