The hotel ballroom was packed to near capacity with scientists when Susan Yanovski arrived. Despite being 10 minutes early, she had to manoeuvre her way to one of the few empty seats near the back. The audience at the ObesityWeek conference in San Diego, California, in November 2022, was waiting to hear the results of a hotly anticipated drug trial.
The presenters—researchers affiliated with pharmaceutical company Novo Nordisk, based in Bagsværd, Denmark—did not disappoint. They described the details of an investigation of a promising anti-obesity medication in teenagers, a group that is notoriously resistant to such treatment. The results astonished researchers: a weekly injection for almost 16 months, along with some lifestyle changes, reduced body weight by at least 20% in more than one-third of the participants. Previous studies had shown that the drug, semaglutide, was just as impressive in adults.
The presentation concluded like no other at the conference, says Yanovski, co-director of the Office of Obesity Research at the US National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, Maryland. Sustained applause echoed through the room “like you were at a Broadway show”, she says.
This energy has pervaded the field of obesity medicine for the past few years. After decades of work, researchers are finally seeing signs of success: a new generation of anti-obesity medications that drastically diminish weight without the serious side effects that have plagued previous efforts.
These drugs are arriving in an era in which obesity is growing exponentially. Worldwide obesity has tripled since 1975; in 2016, about 40% of adults were considered overweight and 13% had obesity, according to the World Health Organization (WHO). With extra weight often comes heightened risk of health conditions such as type 2 diabetes, heart disease and certain cancers. The WHO recommends healthier diets and physical activity to reduce obesity, but medication might help when lifestyle changes aren’t enough. The new drugs mimic hormones known as incretins, which lower blood sugar and curb appetite. Some have already been approved for treating type 2 diabetes, and they are starting to win approval for inducing weight loss.
The ability to melt weight away by tweaking biology gives credence to the idea that obesity is a disease. In the past, scientists and the public often thought that those with obesity simply lacked the willpower to lose weight. But evidence is growing that most people’s bodies have a natural size that can be hard to change. “The body will defend its weight,” says Richard DiMarchi, a chemist at Indiana University Bloomington.
However, some researchers worry that these drugs play into some societies’ obsession with being thin. Body size isn’t always a good predictor of health. “I’m really hesitant to be excited about something that I think is potentially harmful from a weight stigma perspective,” says Sarah Nutter, a psychologist at the University of Victoria in Canada, who specializes in weight stigma and body image.
Research questions abound, including who will respond to treatment and whether people will have to take these drugs for life—a huge barrier to access, given that they also carry a hefty price tag: the injections often cost upwards of US$1,000 each month.
Still, obesity researchers are celebrating these developments. For the first time, scientists can pharmacologically alter weight safely, says physician-scientist Matthias Tschöp, chief executive of Helmholtz Munich in Germany. “It indeed is ‘the’ transformative breakthrough.”
Hormone hunt
The seeds of today’s success were sown decades ago, when Jeffrey Friedman was racing to figure out which gene mutation was making the mice in his laboratory eat until they became obese. In 1994, Friedman, a molecular geneticist at The Rockefeller University in New York City, discovered that the faulty gene encoded leptin, a hormone that is produced by fat tissue and induces a feeling of fullness. Giving leptin supplements to mice that lacked it reduced their hunger and body weight.
“That really revolutionized our thinking about the biological basis of obesity and appetite regulation,” Yanovski says.
An explosion of research into obesity’s underpinnings followed, alongside research into pharmacological treatments. But these early drugs led to only modest weight loss and serious side effects, especially on the heart.
Even before leptin’s discovery, researchers had been looking for hormones that regulate blood glucose levels, and had found one called GLP-1 (glucagon-like peptide 1). It seemed to have the opposite effect of type 2 diabetes—GLP-1 enhanced insulin production and reduced blood sugar—making it an appealing approach to treating obesity, says Jens Juul Holst, a medical physiologist at the University of Copenhagen, who discovered and characterized GLP-1.
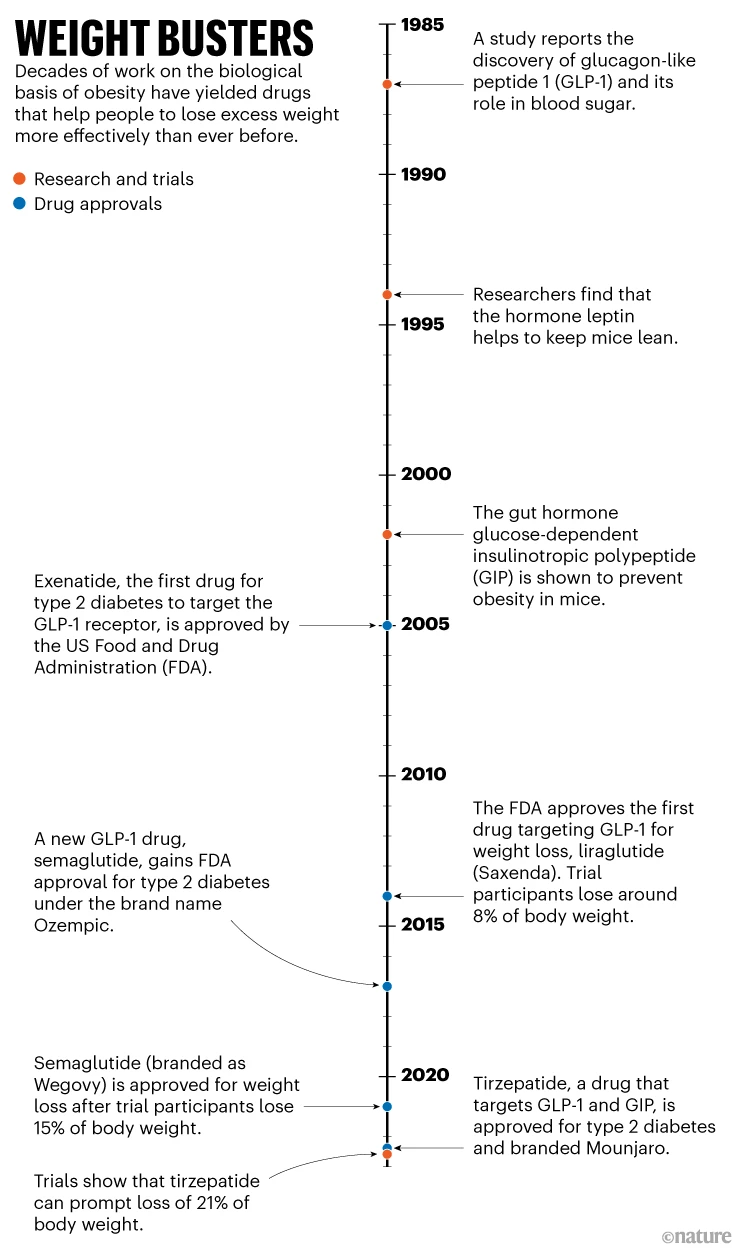
In the 2000s, the US Food and Drug Administration (FDA) began approving drugs that mimicked GLP-1 as type 2 diabetes treatments (see ‘Weight busters’). But scientists noticed that participants in clinical trials also lost weight, owing to GLP-1’s effect on receptors in the brain that govern appetite and those in the gut that slow digestion. Over time, companies began to trial these diabetes medications for weight loss. By the mid-2010s, one such drug, liraglutide, was capable of eliciting a loss in body weight of about 8% on average, 5 percentage points more than for people taking a placebo—clinically relevant, but not astonishing.
But in early 2021, scientists were wowed by a phase III clinical trial investigating a new drug of the same type: semaglutide. The molecule, a modified version of liraglutide, acts on the same pathways but remains intact and active in the body for longer, says DiMarchi. It might also have better access to brain regions that regulate appetite, he adds.
Those receiving weekly injections of semaglutide lost, on average, 14.9% of their body weight after 16 months of treatment; those who received a placebo lost 2.4% on average. In 2021, four years after approving it for diabetes, the FDA approved semaglutide for weight loss for adults with obesity.
Historically, it hasn’t been possible to safely decrease body weight by more than 10% through pharmacological methods, says Timo Müller, a biologist and director of the Helmholtz Munich Institute for Diabetes and Obesity. But these newer treatments also improve cardiovascular health, he adds—the opposite of past iterations.
There could now be an even more effective drug in town: tirzepatide. Tirzepatide doesn’t just target the GLP-1 receptor; it also mimics another hormone involved in insulin secretion, known as glucose-dependent insulinotropic polypeptide (GIP). Approved in 2022 for type 2 diabetes, this treatment—developed by Eli Lilly, based in Indianapolis, Indiana—led to a 21% drop in body weight, on average, at the highest dose, compared with 3% for placebo.
It’s unclear why mimicking both hormones works better than imitating just one. Müller says that tirzepatide might be a more potent activator of the GLP-1 receptor, and that GIP might help to make GLP-1’s side effects more tolerable, allowing for higher doses. It’s also possible that GIP might drive some weight loss on its own.
Despite the uncertainties, the levels of weight loss following tirzepatide treatment approach those typically achievable only through bariatric surgery. This procedure reduces body weight by 30% or more after six months, and the weight loss continues for the next year or two.
“Ten years ago, if you had told me we have something that gets us pretty close [to bariatric surgery], I would have said that’s not possible,” says Ruth Gimeno, group vice-president of diabetes, obesity and cardiometabolic research and early clinical development at Eli Lilly. The company plans to apply for the drug to be approved, pending results from a second phase III trial wrapping up in April 2023.

Mechanism mystery
Despite tirzepatide’s promising results, it has researchers puzzled. It’s clear how GLP-1 helps to spur weight loss, but GIP’s role is a surprise. In fact, scientists have long thought that GIP actually encourages obesity: mice with dysfunctional GIP receptors are resistant to obesity. Therefore, to induce weight loss, researchers thought the receptor should be switched off. But tirzepatide does the opposite.
“We were the first who came up with this crazy idea,” says Müller, who collaborates with Novo Nordisk. “And we were quite heavily criticized in the field.”
Müller and his colleagues—including DiMarchi and Tschöp—knew that GIP stimulates insulin secretion depending on blood glucose levels, just like GLP-1, says Müller. So they developed molecules that mimicked both hormones. After initial studies demonstrated that activating both the GIP and GLP-1 receptors caused weight loss, pharmaceutical companies created their own molecules achieving the same results, thus confirming that the method worked.
A doctor weighs a woman in Spain as part of a weight loss challenge. Diet and lifestyle changes are recommended approaches to weight loss, but a new class of drugs could help. Credit: Miguel Riopa/AFP/Getty
However, not everyone has changed their views on GIP. Holst feels that tirzepatide is simply a super powerful GLP-1 imitator.
It can also mimic GIP, “but it doesn’t really matter in patients with diabetes and obesity, because the GIP part doesn’t really do anything,” says Holst. Eli Lilly is conducting early-stage clinical trials with drugs that target GIP alone, which Holst says will resolve the ongoing debate.
And biopharmaceutical company Amgen, based in Thousand Oaks, California, is pursuing a drug that activates the GLP-1 receptor while thwarting the GIP receptor. Early clinical-trial data show that this treatment reduced body weight by up to around 15% after 12 weeks.
Other approaches include ‘triple agonists’ that mimic the actions of GLP-1, GIP and a third hormone, glucagon, which also stimulates insulin secretion. Still other gut hormones involved in appetite, such as peptide YY, are being explored, too. And some researchers are investigating the monoclonal antibody bimagrumab, which increases muscle mass while decreasing fat.
Open questions
One big question facing researchers now is whether people will need to take these medications for life to maintain their weight. A subset of clinical-trial participants who ceased taking semaglutide and stopped the study’s lifestyle interventions regained about two-thirds of their lost weight after one year.
Another unknown is who will respond to these drugs—and who won’t. It’s too early to tell now, but the drugs seem to be less effective for weight loss in people with type 2 diabetes than in those without. Conditions such as fatty liver disease and having fat around the organs, known as visceral body fat, might also affect how people respond to different drugs, Tschöp says.
Some researchers also worry that by offering a weight solution in societies that prize thinness, these drugs could also inadvertently reinforce the disputed link between excess weight and health. One study found that nearly 30% of people who are considered obese are metabolically healthy. Another showed that other health problems tend to be a better predictor of someone’s risk of death than is weight, demonstrating the need to consider factors other than weight when judging health, says Nutter.
“To pathologize a person’s health simply based on their body weight is potentially really, really harmful,” she adds.
Nutter is concerned that people might start these treatments—whose side effects, such as nausea and vomiting, can be severe—to escape weight stigma, rather than to serve a true health need.
Others worry about the idea that these drugs offer a quick fix. This is a common misconception about bariatric surgery, says Leslie Heinberg, a clinical psychologist at the Cleveland Clinic in Ohio who specializes in bariatric behavioural health and body image. “Some people who still hold on to those mistaken beliefs will say, ‘Oh, now people can just take this pill and that’s the easy way out of obesity,’” she says.
Still, there is plenty of demand. And although these drugs are entering the market, not everyone who needs them will have access.
For a start, they are pricey—semaglutide for weight loss, branded as Wegovy, costs about $1,300 a month—and many insurance companies in the United States refuse to cover the expense, primarily owing to a misunderstanding of what causes obesity and viewing the treatments as ‘vanity drugs’.
“People talk about some of these drugs as being game-changers,” says Patty Nece, chair of the board of directors of the Obesity Action Coalition (OAC), an advocacy group based in Tampa, Florida. But, she adds, “for an individual patient, it’s never going to be a game-changer if they can’t afford it or don’t get access to it”.
Organizations such as the OAC are pushing pharmaceutical companies to offer affordability programmes. Eli Lilly, for example, has a ‘bridging programme’ for Mounjaro—tirzepatide for type 2 diabetes—under which the medication can cost as little as $25 for the first three months. Novo Nordisk has a similar programme for Wegovy.
Whatever the upfront costs, some scientists stress that addressing obesity could allow health-care systems to save enormous amounts of money by reducing a slew of conditions that are linked to the disease.
Although researchers are still chipping away at obesity’s complex combination of causes—including genetics, environment and behaviour—many support the idea that biology plays a significant part. Eating healthily and exercising will always be part of treatment, but many think that these drugs are a promising add-on. And some researchers think that because these drugs act through biological mechanisms, they will help people to understand that a person’s body weight is often beyond their control through lifestyle changes alone. “Tirzepatide very clearly shows that it’s not about willpower,” Gimeno says.
This article is reproduced with permission and was first published on January 4 2023.