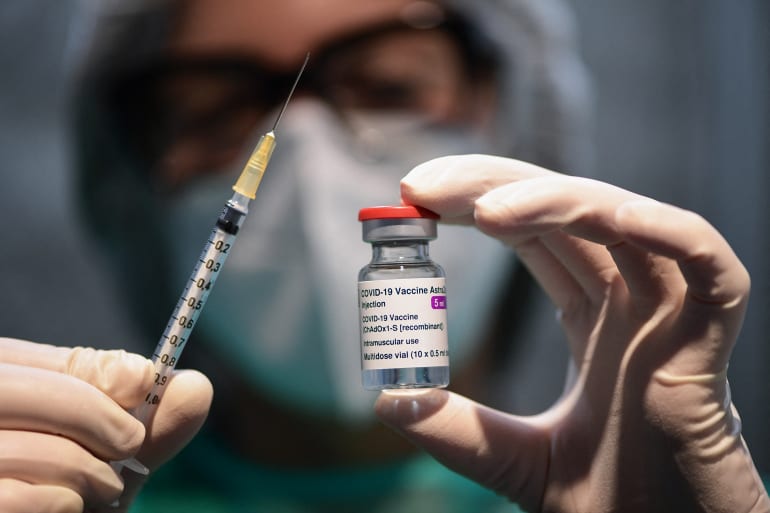
Human body organs are correlated to each other, and unexpected effects of a medicine may occur. It has happened that common medicines which used to be sold without need of a medical prescription have suddenly been banned worldwide.
- Where could one find registered possible unexpected symptoms after the COVID-19 vaccination?
- Almost 500 million people to date have received the different COVID-19 vaccines, out of whom 135 million received also the second dose.
- Research on common as well as rare cases is important, especially for the most adverse cases, as happened when deaths and thrombosis occurred a few weeks ago which led to the suspension of AstraZeneca.
Such was the case, for example, of Ranitidine, a popular heartburn drug discovered in 1976 and in commercial use since 1981 whose withdrawal from the market was requested one year ago by the FDA. In other cases, some medicines are still allowed but their use requires special attention, as in the case of “Tamsulosin,” an usual medicine for the prostate that could affect the eyes, which requires special care when a patient who is taking it must have cataract or glaucoma surgery.
These medicines are actually used by minor fractions of the population. Despite this, a web search for their side effects would easily find accessible references as well as for similar medicines of limited use.
For COVID-19 vaccine side effects, this is not so. This may be is surprising, considering that they are being used by an incomparably larger fraction of the population, which in perspective would actually be almost the entire world population. The information that is provided by the producers is, of course, available, but this is based on the adverse cases occurred during the vaccine test phase. The size of test samples amounts to a few tens of thousands, an order of magnitude less than the almost 500 million people, who until now have received the different COVID-19 vaccines, out of whom 135 million received also the second dose without need to say that these figures are growing day after day as the vaccination campaign progresses.
The broadening of the sample makes possible the appearance of new rare effects that did not arise in the test phase. Besides the most common adverse cases, the effects of low statistical incidence must also be detected and studied. Nevertheless, a simple check in a search engine like Google posing questions such as, “Where can I write my strange symptoms after COVID-19 vaccination?” or “rare symptoms after COVID-19 vaccination” shows that such a website does not exist where one could find registered possible unexpected symptoms after a COVID-19 vaccination.
One can only find a few articles like “What are the side effects of the AstraZeneca vaccine?” “What are the side effects of the Pfizer vaccine?” A quick look at them confirms that besides the most popular symptoms, there are also a few that are rather rare. For example, 4 out of 600 people commented having had bladder problems after the AstraZeneca vaccination, an effect that is not mentioned in the usual lists of possible problems detected during the test phase. Something analogous happens for the Pfizer-BioNTech vaccine. Among more than 200 comments on side effects, 2 of them also report bladder problems, and 15 report a body tingling which in some cases lasted up to 2 weeks.
We are no longer in the 19th or 20th century. The use of receiving results of tests performed in analysis laboratories electronically is widespread. The same procedure could be easily used to collect in a systematic way clear information of unforeseen symptoms or side effects.
It would be enough to create a dedicated website, not necessarily centralized at the country level, since the quantity of vaccinations would justify its statistical usefulness even with a regional management, with an online questionnaire (that can be answered in a few minutes) which allows identifying the major issues: type and batch of vaccine, date and place of vaccination, observed anomalous effect, for example. This databank would allow people to register the observed problem and to put it in relation to similar problems presented with the same vaccine or batch of vaccines for any further investigation that may be necessary.
Research on rare case is not only important for the most adverse cases, as it happened for the deaths and thrombosis that a few weeks ago led to the suspension of AstraZeneca, despite the careful EMA analysis of the cases which led to confirm that at least for the moment a connection with the vaccination is not proven.
The analysis of less serious cases, such as possible rare symptoms useful in particular for the interface and possible conflicts of the vaccine with other medicines that vaccinated people especially the elderly are using, contribute to increasing the general safety of the vaccination programs.
A second benefit that should not be disregarded concerns communication. The case for vaccinating does not require justification, given the tremendous difference between the direct COVID risks and the possible vaccination risks, but the knowledge of the availability of a simple website where problems of unexpected or strange symptoms associated to the vaccination experience are reported bottom to top by vaccinated people and duly checked and analyzed is necessary and urgent, because it will increase the confidence of the population neutralizing as far as possible the fake news about this matter. Last but not least, it will stimulate new research and avoid late understanding of problems, as occurred in the Ranitidine case.
In order to reach this result, a risk must be avoided that against the previously mentioned motivation it may become itself a source and amplifier of fake news. What we are proposing is not a new social site to interchange opinions without a scientific basis, but a site where cases are submitted for analysis by competent specialists.
Galileo Violini co-authored this article.
#rebuildingtravel